Batteries: Types and History
A battery converts chemical energy into electrical energy. The battery was the first device developed to power electrical devices, only later on in the mid 1800's did the dynamo and generator take over as a primary power source. Batteries still occupy an indispensable role everywhere in our lives. They come in all shapes and sizes. Today's engineers are working on exciting advancements, mainly improving energy density and recharge speeds. Improvements in batteries result in very visible changes in society because often it is often battery size, weight and cost which are limiting factors in the advancement of a technology.
|
1.0) Basics |

 |
|
Two ways to classify batteries:
Primary Batteries - this type of battery is ready with electrical charge as soon as it is constructed
Secondary Batteries - this type of battery must be charged after it is constructed
An engineer with a background in electrochemistry or nanotechnology can work on improving batteries and breaking established barriers to improvement. Improving even one angle of performance such as: energy density, low temperature performance, energy storage duration, recharge speed, shape, movement to use of less toxic or less expensive material can create significant changes to our world. For example the hybrid and fully electric automobile has existed for a century, but it was better batteries that allowed the first mainstream use of EVs in the 1990s.
1.a) Types of Batteries
There are many ways to make a battery, some models are over 200 years old and others (such as those using carbon nanotubes) are being quickly advanced right at this moment!
|
|
|
2.) History of Batteries
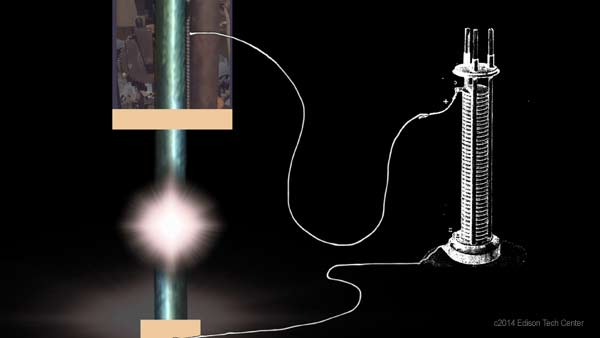
Above: illustration of a volta battery powering an experimental arc lamp, the first form of electric lighting
2.a) 1800: Voltaic Pile - the first battery directly causing the electrical revolution
 Alessandro Volta of Como, Italy created the first modern battery around 1800. He had a background of chemistry and physics and taught at a public school and later the Royal School in Como. He used a zinc and copper (or silver) electrode with an electrolyte of sulphuric acid or a brine mixture (salt and water). The zinc reacted with the negatively charged sulfate. The positively charged hydrogen ions grab electrons from the copper forming hydrogen gas. The zinc disk then became the negative electrode and the copper/silver disk positive. Volta's first battery was a result of 9 years of study starting with "animal electricity" or the study of electrical current within the body. Like all great innovators he advanced the work of those before him, in this case it was Luigi Galvani and his work on 'animal electricity'. > |
|
Volta's battery quickly captured the attention of the world. Researchers from Russia to the US began experimenting with versions of his battery to conduct experiments. Electroplating, separation of elements for scientific study, electric lighting, and electromagnetism studies all advanced rapidly thanks to Volta's pile. It was called a 'pile' because additional zinc/copper units could be stacked onto the device to increase power. Even today latin languages use the word "pila" to mean battery.
Flaws of volta's battery were that the brine soaked cloth material needed to stay moist, also the electrolyte leaked down the side and caused shorts. Chemical build up on the copper caused an insulative layer which stopped the battery after about one hour. Over the next three decades others like William Sturgeon and Joseph Henry improved the batteries design.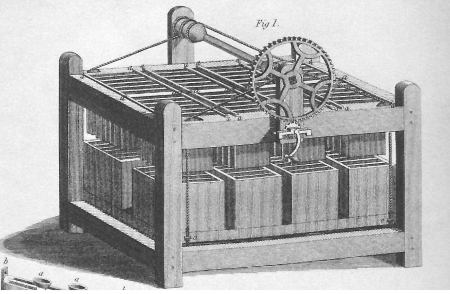
Above: Joseph Henry's Galvanic Battery (made of zinc and copper plates) was designed to produce a varying intensity depending on the need by using a set of movable connectors and cups of mercury. Today the device we'd use to provide changing power levels for an experiment would be the variac (autotransformer) attached to grid power. Henry needed the variable power levels to conduct his experiments on electromagnetism.
Read more detail about Henry's battery from this scan from Princeton >

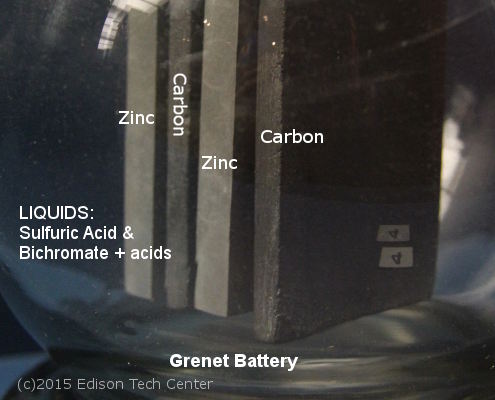
2.b) 1857-1900s Grenet Cell "The Bottle Battery"
The Grenet Cell was an important step in battery history and was used for over 60 years. This wet cell was powerful and reliable. It was filled with acid and could be reused. By the 1880s units could last months or years without a refilling, although for some uses it needed refilling every few weeks. Thomas Edison used Grenet Cells for his experiments, medical professionals also used the cell to do everything from powering tools at hospitals to experimentation on patients. The cell came in various sizes, the flasks could also be placed in series to generate more power.
The end of the Grenet Cell came from its weaknesses which included the annoyance of refilling it, weight, and the fact that it could spill. The rubber sealed board (hydrostat) would shrink with time and enable leaks to happen out the top. Like many batteries at the time it was made of glass and while it was built with thick glass, it could still shatter.
The Grenet Cell was improved by Dr. Byrne (Brooklyn) in 1878. Modern dry cells today also use the zinc-carbon cell, however they use a moist cardboard for the electrolyte instead of the Grenet Cell's liquid acids.
The Lead Acid Battery
1859 - Gaston Plante of France invents the most commonly used large battery today: the lead acid battery. More on the lead acid battery >
Today's lead acid batteries (found in your car for example) have an energy density of about 30 watt hours per kilogram.
2.c) The Dry Cell
In 1886 dry cells were developed and this was a huge improvement for some applications of the battery. The dry cell used a paste electrolyte and this allowed the battery to be used in any orientation and improved the area of portable batteries. Karl Gassner and developed the dry cell using plaster of paris mixed with other chemicals. The first model for sale produced 1.5 volts. Later on the plaster of paris was replaced with a coiled cardboard. Columbia produced the first mass produced models.
Below left: classic 1.5 V Columbia dry cells on display at the Edison Tech Center.
Below right: 3 classic dry cells as used in an early radio.


For lithium, alkaline and other modern forms of dry cell batteries see below.
2.d) Thomas Edison and Batteries
Thomas Edison had a focus on making a better battery for use in electric vehicles. Existing batteries like the Grenet Cell were made of glass and inadequate. Edison made his mark in the world of batteries with many improvements. The Edison-Lalande cell was a landmark improvement in batteries, it had improved durability and lasted about one year in storage. Edison had a long time interest in batteries of all sizes to power his inventions such as the electric pen. Edison's last major improvement to batteries was the development of a practical iron-nickel battery (NiFe). The early Edison NiFe batteries used thick glass housing to hold the potassium hydroxide electrolyte. Some models of these batteries could hold charge for many years. The railroad industry still uses decades-old NiFe batteries to back up switches and other apparatus due to its long term reliability.
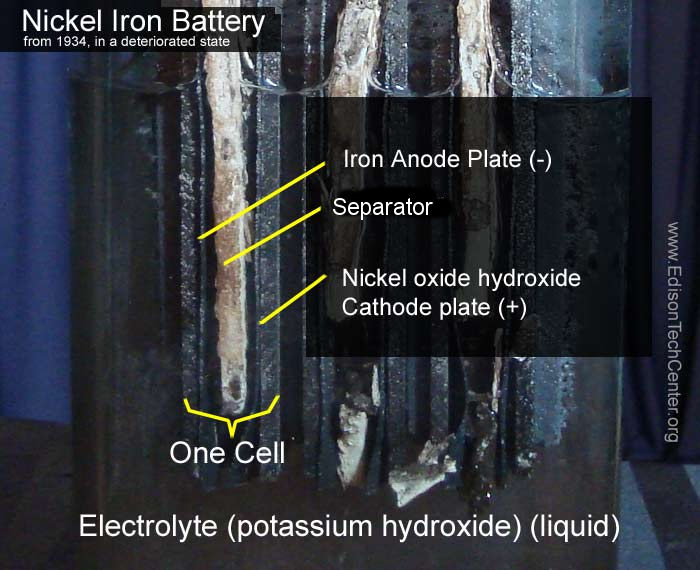



Above: Original Edison batteries used in the 1914 Detroit Electric car owned by Steinmetz

The AA alkaline is the most
common disposable battery in the world
3.) Modern Batteries:
Today the world is dominated by zinc, lead, and lithium based batteries. They are safer and smaller than the same type of batteries were in the early days. Mercury and other chemicals have been reduced in the composition, and the energy density improvements over the years have made for the use of less material per watt.
The Alkaline Battery:
Alkaline batteries are the most common disposable battery today (10 billion units produced
world wide each year). They use zinc and
manganese dioxide. The alkaline has replaced the zinc-carbon battery of the 1800s due
to higher energy density.
Problems with alkalines include leaking potassium hydroxide (the white feathery crystals seen
when a battery becomes old).
The alkaline battery is made of cheap materials so it is not cost effective to recycle it, as
a result it is thrown into mainstream waste leading to
increased toxic waste in landfills.
3.a) Lithium-ion Batteries (LIB)
Lithium batteries are currently the most popular battery for mobile applications
(vehicles, hand-held devices)
due to low weight and great energy density (amount of energy you can store per
kilogram of weight). Lithium batteries come in different forms:
 |
Lithium iron phosphate (LFeP) - 120+ watt hours per kilogram |
 |
Lithium cobalt oxide battery (LiCoO2) - 100+ watt hours per kilogram, used in mobile phones and other smaller devices. This type of battery is used in laptops for its high energy density, problems include thermal runaway which can cause fires. |
 |
Lithium Titanate (LTO) - They are safer than other forms of lithium batteries (less chance of thermal runaway). They have a 10-20,000 cycle life and 70-80 watt hours per kilogram (3x the capacity of the standard lead acid battery). |
See our video here with Testing Lithium Batteries in Dr. Andy Burke's Lab: Three Types of Li Batteries:
Nanotechnology will improve lithium-ion batteries: Carbon nanotubes can be used as a cathode and this allows for lithium storage reaction on the surface of the tube, which is much faster than conventional lithium intercalation reactions. Read more here >
4.) Frontier of Battery Innovations
Tesla Motors, General Electric and others are in a race to develop better and cheaper batteries. New battery developments such as the sodium-ion, sodium-nickel-chloride (part of the GE Durathon battery brand) offer promise to replace large scale lead-acid batteries used in energy grid and locomotive applications.
For batteries to really succeed in electric vehicle applications experts have set out goals that batteries will need to last through 15+ years of deep discharges and be able to be recharged as quickly as a gasoline fuel tank can fill with fuel. These are tough goals but it is being worked on now. Using nanotubes in the lithium battery it may be possible to recharge the battery much faster, however ensuring a longer life cycle will be a harder challenge. Read more here >
5.) Pre-electrical age batteries:
It is worth mentioning that there may have been batteries prior to the modern electrical age. Since these are disconnected from the main timeline of electrical history we have listed them here.
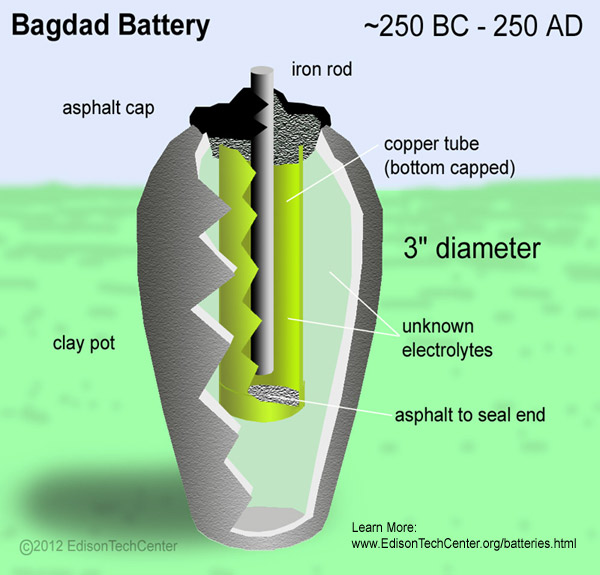
The first battery 248 BC: The Bagdad Battery was built in the Parthian or Sassanid period ~248 BC - 226 AD. The battery consisted of a carbon rod in the center of a clay vase. The rod was surrounded by an unknown electrolyte (likely to be orange/lemon juice), then copper, then asphaltum. Each battery had a weight of about 2 kilograms and produced 0.4-0.5 volts with open contacts. These batteries were very weak. The "Bagdad Battery" was found in 1936 and is believed to be authentic by many reputable sources.
The Egyptians: Some claim that the ancient Egyptians had batteries similar to the Bagdad Battery.
The Ark of the Covenant: It has been theorized that the Ark of the Covenant (a gold lined box) may have used early batteries to energize the gold exterior. The box would then be able to give the illusion of magic powers by shocking those who touched it. This is only a theory, but would be an interesting use of electricity to create a sense of awe and fear.
6.) ETC Videos featuring batteries:
The Tesla Model S - batteries, body and suspension >
The Hybrid Vehicle Battery Lab with Andy Burke >
History
of the GE battery business by Dr. Oliver Winn
(former manager) >
The Baker Electric Car used Edison lead-acid batteries in 1901 >
The first computerized hybrid vehicle HTV1 (lead acid batteries) 1978-1982 >
Related Topics:
|
Diesel Electric Locomotives |
Electric Cars |
Photovoltaics |
Capacitors |
Semiconductor Electronics |
More Stuff |
Article by MW
Sources:
The Telegraph and Telephone Age. By D. McNicol. 1915
Santa Clara University
Rutgers University: The Thomas Edison Papers
The New York Medical Journal, Jan-Jun 1889
Progressive Dynamics Inc.
Energizer
Wikipedia
IEEE Spectrum
General Electric
A Treatise on Nervous and Mental Diseases by Landon Carter Gray. 1893 Corrosion-doctors.org
Dr. Andrew Burke. University of California at Davis. 2010
Photos/Video:
Edison Tech Center
Whelan Communications
For use of Edison Tech Center images and videos see our licensing agreement.


